Can Parkinson's be stopped by unravelling protein fibres? Anne Wentink finds out with a Vidi grant from NWO
In brain diseases such as Parkinson's and Alzheimer's, proteins clump together to form fibres. ‘Chaperone proteins’ unravel those fibres, but in the test tube biochemist Anne Wentink saw that this can also cause new problems. She is going to find out what happens inside cells to determine what a drug should do.

Anne Wentink is fascinated by chaperone proteins. 'Their name refers to the Victorian era, when well-off young people were always accompanied by chaperones to keep them from missteps. In a similar way, chaperone proteins guide newly formed proteins as they are produced and fold. They also ensure that they maintain the correct 3D structure during their lifetime.' Other proteins often respond very specifically to one particular receptor and then do their job. Chaperones are different. 'They are unique among proteins because they work so aspecifically and yet are effective.'
Thus, when chaperone proteins encounter the clumped fibres typical of neurodegenerative diseases such as Alzheimer's, Huntington's and Parkinson's, they spring into action. They pull the fibres apart into the single proteins and in this way can counteract the harmful deposits. During her postdoc research, Wentink and her colleagues studied in a test tube what happens when chaperone Hsp70 attacks the protein fibres. 'We saw that the loose fragments arising from the broken fibres easily form new fibres again. This could accelerate the disease. We wonder if this also happens in cells, or if there are other mechanisms there that counteract it.'
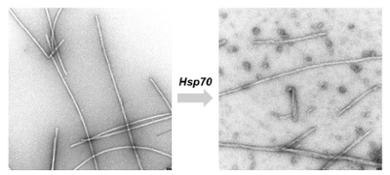
First in test tube, now in the cell
The balance between positive and negative effects of Hsp70 is what Wentink now wants to know more about. With NWO's Vidi grant, Wentink is therefore moving her research from the test tube to living cells. 'We produce fluorescent protein fibres and introduce them into cells. Then we watch how the breakdown process proceeds.' For this, they will use microscopy and flow cytometry: a technique that uses laser light to count and study small particles in a flowing fluid. 'We manipulate the amount of Hsp70 chaperone in cells and see what happens when there is more or less of it. We also apply fragments from an unravelled fibre onto other cells to see if this cause fibres to form rapidly there or not at all.'
Cause or consequence
Wentink also makes a side note: ‘It is not certain whether the aggregated protein fibres are cause or consequence of brain diseases. We do know from brain research that they are characteristic of these diseases. In Alzheimer's, they are fibres of amyloid-beta and the Tau protein, in Parkinson's α-synuclein.' Wentink studies fibres from the latter protein, but the knowledge she gains is also relevant for other neurodegenerative diseases.
If it turns out that the amount of chaperone matters a lot for the onset and spread of Parkinson's fibres, a synthetic chaperone might be an effective drug. 'In the second part of my research, we are going to develop such a synthetic chaperone,' says Wentink. 'That has to be a molecule other than Hsp70, because that does much more in cells than just breaking apart these protein fibres. We need a molecule that specifically targets these fibres.'
What will be the drug target?
Perhaps Wentink discovers that the negative effects of Hsp-70 in the cell are not so bad. 'It could also be that this protein is not the only mechanism in the cell that breaks down protein fibres, but even then we may be able to accelerate these processes via Hsp70. 'Our project is an important step in neurodegenerative disease research. It helps determine which target a future drug should act on.'
Unintentional protein aggregation and chaperone Hsp70
Imagine a protein molecule as a ball of wool, consisting of a chain of atoms with all kinds of loops in it. It is loosely coiled and moves around inside a cell, through a watery environment. This works best if the water-soluble or hydrophilic pieces are on the outside and the hydrophobic, oily parts on the inside. If a protein is not folded properly, such a hydrophobic part almost always ends up on the outside. These sticky surfaces can stick together, leading to protein aggregation. If a chaperone protein such as Hsp70 encounters this, it starts to pull on the loosely coiled tangle. This unravels, and can then spontaneously fold back into the correct 3D structure.
Text: Rianne Lindhout
Image above article: Pixabay
