Superselective bonds light up
Rather than one key and one strong lock, biology often uses tens or hundreds of weaker links to bind parts together, such as cells membranes. This allows for selectivity and also reversibility: the binding can also be undone. Researchers first caught this phenomenon using spheres or colloids, and published today in the journal PNAS.
Binding is everywhere in biology: the way an immune cell recognizes an invader, the way a virus latches on to its victim cell, the way drugs bind to their target cells. In each case molecules on the surface of one party fit into the molecules of the other side, to the two sides recognize each other, and connect, for good or for bad.
This molecular recognition has often been likened to a key fitting into a lock, but according to Christine Linne, a researcher at Leiden and Delft Universities, in many cases velcro is more appropriate metaphor. ‘We are looking into multivalent binding: instead of one one molecule that binds strongly to one receptor, there are tens or hundreds of thousands of weak links.’
This is important for many biological interactions. For instance, just as velcro sticking can be easily undone, multivalent weak binding is reversible.
Superselectivity
A surprising property of these multiple weak bonds is that they can be very sensitive to the density of receptors and the molecule that fit into them: when there are few receptors, there is no binding, but when the receptor density reaches a certain threshold, the chances of the colloids to bind increase drastically.
‘When this transition is sharp, this is called superselectivity’, says Linne, ‘and it may play a role in drugs targeting cancer cells. Often, these cells have the same receptors on their surface as normal cells, but in much higher densities. Superselectivity could allow a drug to bind to the latter, densely populated cancer cells, but not to the former, normal cells.’
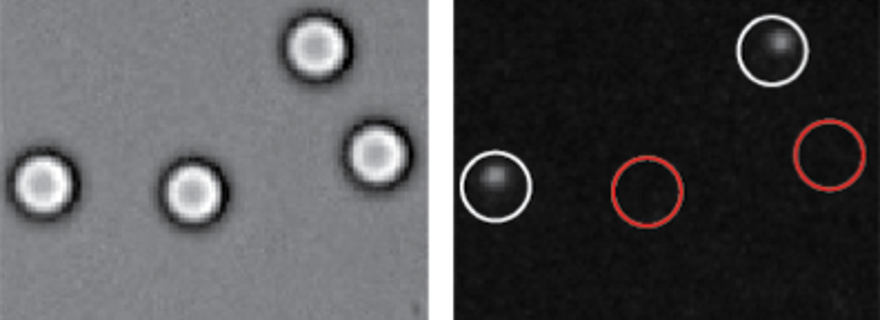
In order to study superselectivity, Christine Linne and colleagues experimented with micrometer sized glass spheres, also known as colloids, with DNA strands attached to them. The colloids are resting on a surface which complementary DNA strands attached. These match the colloid DNA like a lock and key, so the colloids can bind to the surface.
Bleaching the dye
But Linne found that they do not necessarily do so. ‘At low densities, the colloids do not bind to the surface. Then, we addded more DNA to the surface, so the density increases. At one point, there is a sudden very sharp incline in the number of colloids that do bind’, says Linne. This is the sought-after superselectivity.
Linne and colleagues uses a technique called FRET (Foerster Resonance Energy Transfer) to make the binding visible. ‘Each DNA molecule also has a dye molecule attached to it, with different colors. When two DNA-strands are bound together, the dye molecules are also very close. So if you excite one of the dye molecules with a laser beam, energy will be transfered to the other one, which will light up, too.’ By imaging this other color, you can distinguish between bound and unbound colloids.
This clever technique also helped the researchers to show another property of the weak binding: reversibility. ‘When you apply an intense laser, you can bleach the dye molecules, which basically means destroying them’, explains Linne. So they shone this light on a bound colloid, destroying all dye molecules. Naturally, the FRET technique did not work anymore, and the bound colloid did not light up anymore. But with time, some of the FRET-light started to return steadily.
Medical Applications
‘This means that the binding is a dynamical, reversible process’, says Linne, ‘DNA molecules can move in and out of the binding patch, so some of the new ones will move out, while others start to contribute to the binding while they still have working dye molecules. This is what we were seeing.’
It is the first time that this reversibility has been shown in this way, and Linne and co-researchers wrote an article in the Proceedings of the National Academies of Science (PNAS), appearing today. ‘Colloids are a new way to study this phenomenon’, says Lnne
Superselectivity, being such a common phenomenon in real biology, could have its applications in medicine, for instance in a system for targeting drugs accurately, or in testing applications.
